As the first step towards a future where anyone who develops Type 1 Diabetes can freely choose from multiple methods to cure Type 1 Diabetes, we aim to realize bioartificial pancreatic islet transplantation as one of the curative methods in 2025.
We appreciate your support.
Donations to Japan IDDM Network are eligible for taxbenefits
※Following sites are only available in Japanese.
Donation deductions and tax benefits About receipts
From insulin replacement to pancreatic islet replacement (bioartificial islet transplantation)
Bioartificial islet transplantation: A day-care approach to islet transplantation for Type 1 Diabetes patients who wish to undergo the procedure
The goal of bioartificial islet transplantation
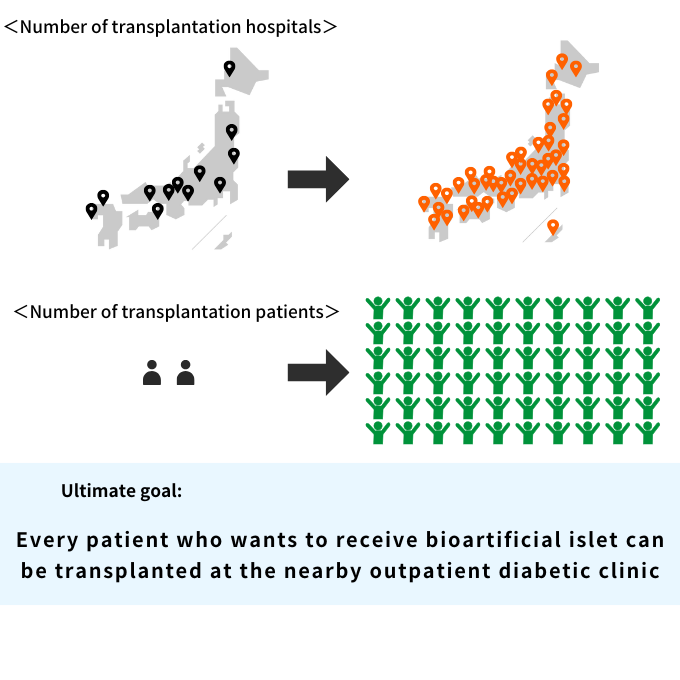
- Any Type 1 Diabetes patient who wants to receive islet transplant should be able to receive it.
- No need for immunosuppressants
- The procedure can be performed on an outpatient basis at a surgery department with a diabetes department, reducing the burden on patients.
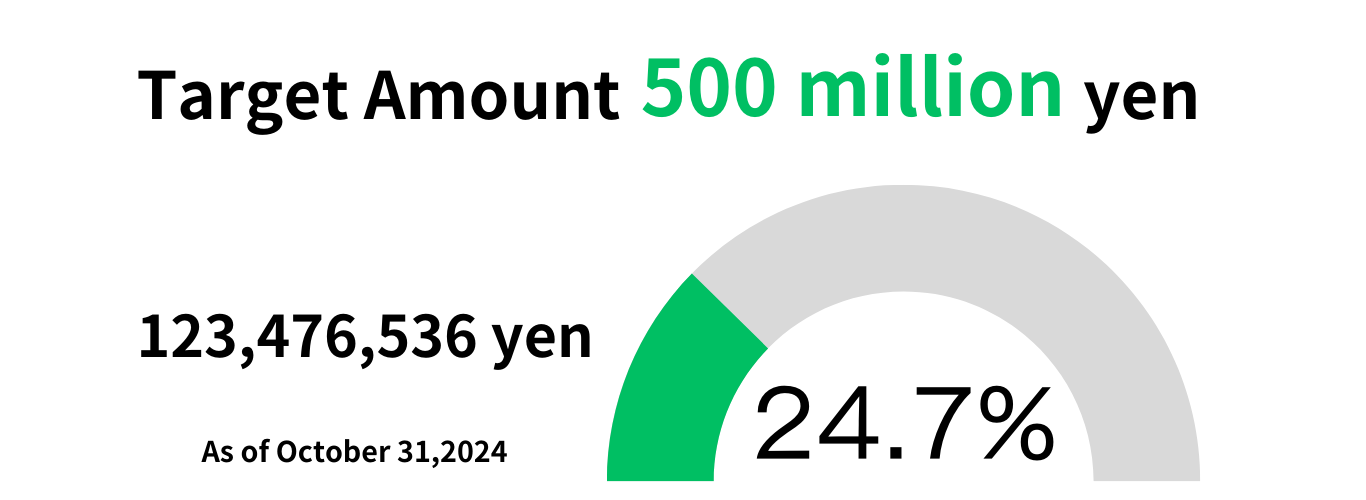
Donations to support the realization of bioartificial pancreatic islet transplantation
Target Amount
What is bioartificial islet transplantation?
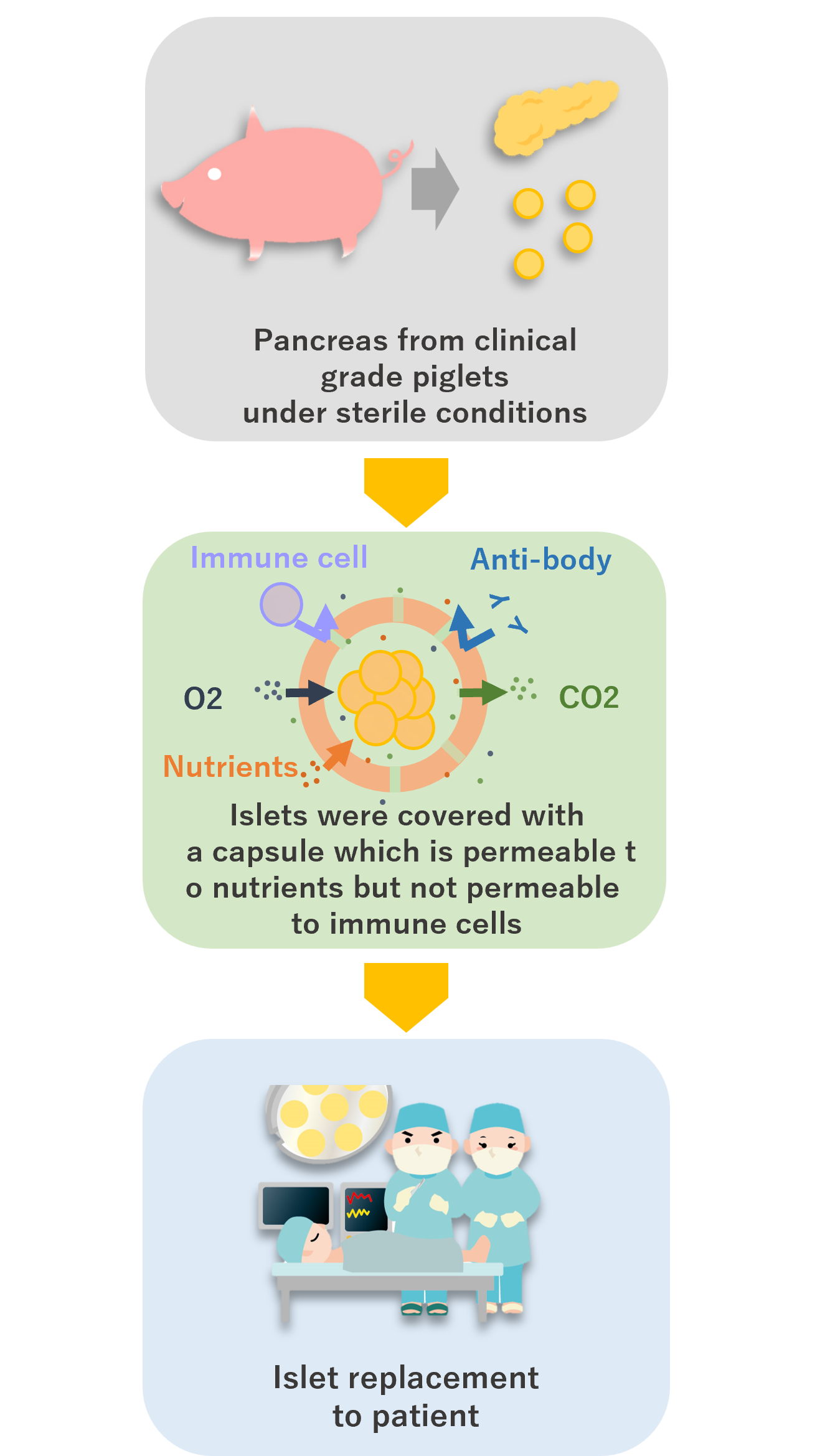
Pancreas transplants and pancreatic islet transplants are covered by health insurance as a method to cure Type 1 Diabetes, but there is an overwhelming shortage of donors in Japan.
Bioartificial islet transplantation is being considered as a solution to this donor shortage.
Bioartificial islet transplantation involves encapsulating pancreatic islets from medical grade pigs raised in a sterile room and then transplanting them into the patient.
Donations to support the realization of bioartificial pancreatic islet transplantation
Benefits of bioartificial islet transplantation
① Less strain on the body
In bioartificial islet transplantation, pancreatic islets (islets of Langerhans), which are clusters of endocrine cells (cells that secrete hormones) found in the pancreas, are transplanted. Because islets are very small, the surgery is smaller than in the case of organ transplantation, and the burden on the body during transplantation is lighter.
② No need for immunosuppressants
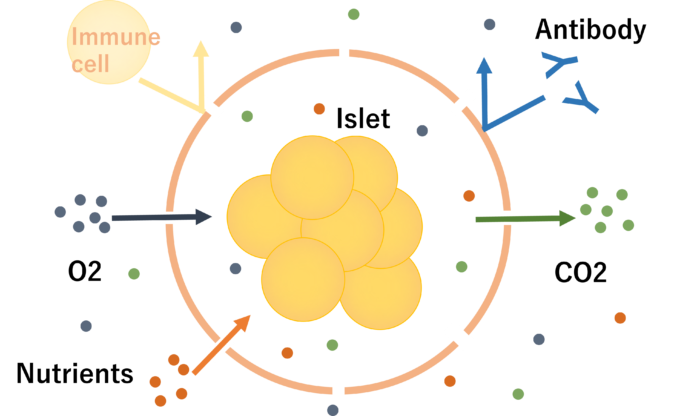
In bioartificial islet transplantation, medical grade pig islets are wrapped in a special capsule. This capsule has very small holes that allow small substances such as nutrients and oxygen to pass through, but larger substances such as immune cells and antibodies (both of which have the function of eliminating foreign substances other than one’s own cells) cannot pass through. Therefore, the transplanted islets are not attacked by immune cells, so there is no need to take immunosuppressants.
Donations to support the realization of bioartificial pancreatic islet transplantation
To patients, their families, and supporters
Dr. Shinichi Matsumoto’s Diabetes Cure Plan(Japanese)
We asked Dr. Shinichi Matsumoto, Japan’s first islet transplant doctor and a leading expert in bioartificial islet transplantation, about “Establishing a cure for Type 1 Diabetes by 2025.”
 Visiting Professor, Kobe University
Visiting Professor, Kobe University
Director, Medical Porcine Development Organization
Graduated from Kobe University School of Medicine in 1988.
Studied abroad at the University of Minnesota in the US in 1997 and the University of Washington in 1999, where he engaged in research and clinical practice in islet transplantation.
From 2002, he engaged in research and clinical practice at Kyoto University Hospital, and in 2004, he successfully performed Japan’s first human islet transplant.
From 2007, he engaged in research and clinical practice at Baylor University Hospital, and since 2012, he has been working on the “Islet Transplant Project” with the aim of curing Type 1 Diabetes.
Q:What does “establishment of a cure for Type 1 Diabetes” mean in 2025?
A(Dr. Matsumoto):Capsulating pig islets and transplanting them into humans for the first time in the world.
Q:Doesn’t this mean that patients who want to receive transplants will be able to do so right away in 2025?
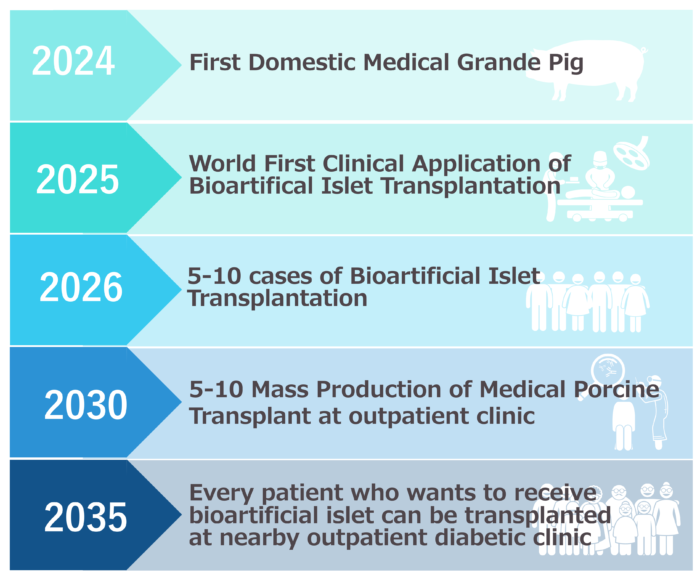
A: We aim to perform 5-10 cases of bioartificial islet transplants within one year from 2025, and to obtain time-limited approval from the government (proof of safety and effectiveness), which is equivalent to a “provisional driver’s license.” Once this is achieved, companies will be able to manufacture and sell encapsulated pig islets.
Q:When do you think you will be able to realize your goal of performing islet transplantation as a same-day outpatient procedure, and when will all patients who wish to receive a bioartificial islet transplant be able to receive one?
A:We would like to realize this in the early 2030s, by pooling the efforts of patients, their families, medical professionals, researchers, the Ministry of Education, Culture, Sports, Science and Technology, the Ministry of Health, Labor and Welfare, the Ministry of Economy, Trade and Industry, companies, politicians, and the Japanese people. In order to make it a same-day outpatient procedure, we are aiming for a method that allows the transplantation to be performed subcutaneously under local anesthesia.
Q:Will additional islet transplants become unnecessary after one bioartificial islet transplant?
A:When human allogeneic islet transplants were performed at Baylor University, it became possible to wean patients off insulin after one islet transplant. We are aiming for a similar effect.
On the other hand, in the case of bioartificial islet transplants, there is no problem of a shortage of donors, so our current goal is to:
・Finish with one transplant without islet function declining
・Can perform additional transplants without waiting if islet function declines
Masayuki Shimoda Director, Islet Transplant Center, National Center for Global Health and Medicine Hospital/Project Director, Islet Transplant Business Collaboration Project, National Center for Global Health and Medicine Research Institute

This project aims to apply the new technology of “bioartificial islets” using porcine islets to clinical applications. The goal is to develop a groundbreaking transplant treatment for Type 1 Diabetes patients that has the potential to eliminate the shortage of donors and eliminate the need for immunosuppressants. Ultimately, we aim to develop a product that is so effective that insulin injections will no longer be necessary.
Furthermore, we are looking ahead to treating Type 2 Diabetes patients with insulin deficiency.
In recent years, research on “bioartificial islets” has progressed significantly, and clinical studies and animal experiment results showing effectiveness are accumulating. In addition, the legal environment for clinical application is being established, making it more feasible. There are still various hurdles to overcome before it can be used clinically, but we will continue to persevere and steadily advance our research, including the development of the necessary facilities, with the aim of realizing this by 2025.
Tatsuo Inoue, Chairman of the Japan IDDM Network
 We hope that this “bioartificial islet transplantation” is the closest thing to a “cure” for Type 1 Diabetes, and our immediate goal is to transplant the first “bioartificial islet” into a human in Japan in 2025 (clinical trial).
We hope that this “bioartificial islet transplantation” is the closest thing to a “cure” for Type 1 Diabetes, and our immediate goal is to transplant the first “bioartificial islet” into a human in Japan in 2025 (clinical trial).
And beyond that, we aim to make it possible for all patients who wish to receive a transplant on a day trip by 2035.
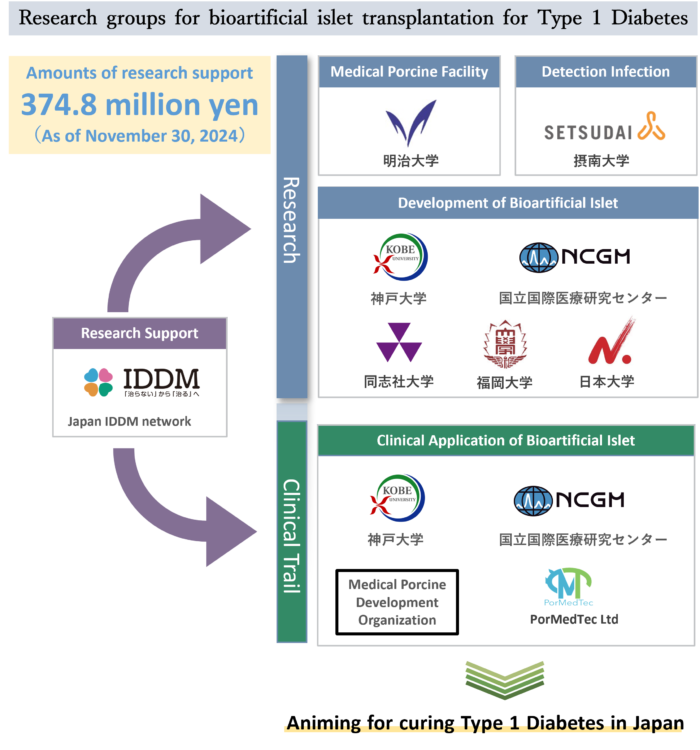
In addition to the research and development centered on universities and other institutions, we are now moving toward mass production (industrialization) of bioartificial islets with the participation of companies.
We, the Japan IDDM Network, have built an all-Japan system that connects these various research institutions and companies, and are promoting this project as the driving force behind it, with funding from the “Bioartificial Islet Transplantation Japan Protocol 2025 Fund.”
As the representative of the Japan IDDM Network and as the father of a patient, I will do my best to make this dream come true.
We would appreciate your support and cooperation in realizing bioartificial islet transplantation in 2025 and toward our goal beyond that.
Donations to support the realization of bioartificial pancreatic islet transplantation
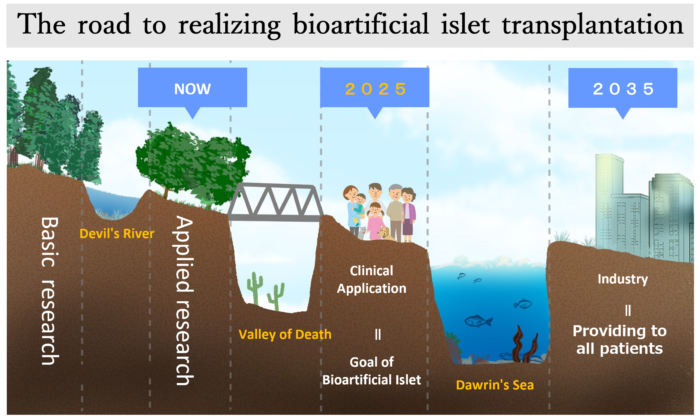
The road to realizing bioartificial islet transplantation
*In the process of researching new technology and establishing it as an industry, the difficulty of moving from basic research to applied development research is referred to as the ” Devil’s River ,” the difficulty of moving from applied research to commercialization is referred to as the ” Valley of Death ,” and the difficulty of the product being established as an industry and becoming a main treatment is referred to as the ” Darwin’s Sea .”
How to make a one-time donation (by credit card)
We currently accept the following credit cards:

Please click the button below to proceed to the donation payment page.
How to make a monthly donation (by credit card)
We currently accept the following credit cards: